Trending Now
MOST RECENT ARTICLES
TRENDING NOW
Dental Radiology: New Recommendations for Patient Lead Shielding
Radiographs are an important aspect of providing comprehensive patient care in dentistry. Though many patients balk at the idea of getting radiographs, it’s imperative...
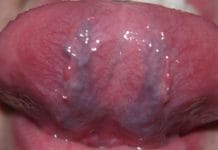
Caviar Tongue: Are Dental Hygiene Patients Displaying Signs of “Aging?”
Caviar tongue is a condition recognized by purplish veins located on the ventral side of the tongue. Veins are normally visible underneath (ventrally) the...
Dental Polishing and Cleansing Agents: A Quick Guide to Coronal Polishing Considerations
The concept of polishing was noted in Roman and Greek writing. It was not until Pierre Fauchard, the father of modern dentistry, associated polishing...
4 Simple Tips for Newly Graduated Dental Hygienists
Disclosure: This article has been sponsored by Heartland Dental, but Heartland Dental had no input in the editorial process or writing of this article.
Congratulations!...
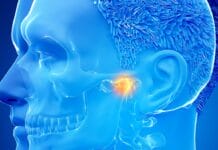
A Dental Hygienists’ Overview of Temporomandibular Joint (TMJ) Replacement
Total joint replacements are surgical procedures that replace a damaged or arthritic joint with a prosthetic joint. This procedure is common knowledge among the...