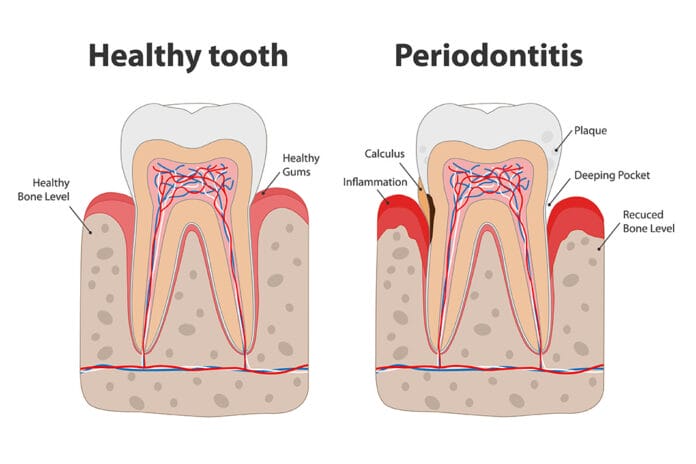
When I graduated from dental hygiene school in the 1980s, the central idea of periodontal disease development and progression was that only specific periodontal pathogens cause periodontal disease and that all other bacteria present in the oral cavity are either beneficial or benign. It was also believed that the bacterial load must reach a certain threshold, at which point the host’s ability to neutralize the bacteria would be overcome.
Additionally, it was believed that one or more of the “red complex” bacteria (Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia) must be present in the biofilm colony for destruction to occur.1 We also knew that host-specific, genetic, environmental and lifestyle factors, such as tobacco use, poor nutrition, diabetes, and stress, resulted in impaired immune response and influenced the development and progression of periodontal disease.2
Armed with this knowledge, we went out into practice. One of the dangers of believing in this periodontal disease development model was that we accepted the idea that we had plenty of time between the onset of gingivitis and the progression to periodontitis to “watch” the condition. We got into the habit of watching and waiting while we tried to educate and encourage our patients to improve their self-care techniques and habits before the level of pathogenic bacteria built up to sufficient numbers to cause destruction.
We used that perceived time lag to encourage our patients to floss, and we would check them again at the next appointment to see if the inflammation had resolved. Almost universally, it hadn’t. This same routine would be repeated again and again until the inflammation almost inevitably progressed to attachment and bone loss, and, at that point, we would treat the periodontal disease.
A Concerted Effort by Keystone Pathogens
In 2012, I was skimming PubMed in search of a topic for an article when I came across a study by Hajishengallis and Lamont.3 They wrote:
“Recent advancements in the periodontal research field are consistent with a new model of pathogenesis according to which periodontitis is initiated by a synergistic and dysbiotic microbial community rather than by select ‘periopathogens,’ such as the ‘red complex.’ In this polymicrobial synergy, different members or specific gene combinations within the community fulfill distinct roles that converge to shape and stabilize a disease‐provoking microbiota. One of the core requirements for a potentially pathogenic community to arise involves the capacity of certain species, termed ‘keystone pathogens,’ to modulate the host response in ways that impair immune surveillance and tip the balance from homeostasis to dysbiosis. Keystone pathogens also elevate the virulence of the entire microbial community through interactive communication with accessory pathogens.”
My interpretation of this research is that the entire colony of multiple different bacteria (polymicrobial) in the oral microbiome works together (synergy) to initiate a microbial imbalance (dysbiosis), resulting in periodontal disease and destruction. The concerted action of the entire bacterial colony is exponentially greater than the action of the individual microbes. This creates an imbalance in the microbial flora leading to inflammation and periodontal destruction.
This process of increased pathogenesis is accomplished by interbacterial communication primarily as a result of the presence of the keystone pathogen, Porphyromonas gingivalis. This keystone pathogen elevates the entire bacterial colony’s virulence by altering the gene expression of the other bacteria, rendering them more aggressive. Porphyromonas gingivalis flips the switch that converts other bacteria, previously thought to be benign or beneficial, into destructive microbes that contribute to periodontal disease progression.4 Research shows that Porphyromonas gingivalis also impairs host immunity, decreasing the ability to neutralize the bacteria, enabling the microbial community’s survival.5
An Early Sense of Urgency
The concepts of keystone pathogens and the polymicrobial synergy and dysbiosis model have significant ramifications for the development of therapeutic options for periodontal disease. With the old model, there was little urgency to treat periodontal disease in the early stages. We believed increasing numbers of periodontal pathogens were required for periodontal destruction to occur. Now we know if even a low level of Porphyromonas gingivalis (or other keystone bacteria yet to be identified) is present, the entire oral microbiome will become more pathogenic.1
This means that even in cases of early-stage gingivitis, we should make concerted efforts to eliminate the inflammation and restore the microbial biofilm back to homeostasis. Identifying the bacteria present in the oral microbiome using salivary diagnostics will assist efforts to control those bacteria before the disease progresses to full-blown periodontitis.6 No longer do we have the luxury of time when it comes to treating gingivitis and possibly preventing periodontitis.
Not only do we have the threat of periodontal destruction, but we also know that, with gingival inflammation, the risk of extensive systemic sequelae increases. Gingivitis is a precursor to heart attack, stroke, pregnancy complications, dementia, rheumatoid arthritis, bacterial pneumonia, certain cancers, and many other systemic inflammatory conditions.7
Early Intervention is a Game-Changer
Once I grasped the significance of the polymicrobial synergy and dysbiosis model, my practice completely changed. Looking back over the years, I treated patients based on the old model, and I shudder to think how many people with gingivitis had serious systemic diseases and conditions that I never knew were related to their oral inflammation.
The fact that we could potentially prevent devastating medical conditions with early intervention in gingivitis treatment changed everything for me. I was no longer willing to wait and watch my patients’ oral and overall health deteriorate. I developed my personal treatment philosophy of care centered around this new model of periodontal disease and the need for early intervention to prevent systemic illness.
I would treat at the very first sign of inflammation. That doesn’t mean that I would perform full-mouth periodontal therapy at the first sign of bleeding, but that was the point at which I would do something different ─ no more watching and waiting. I decided that my treatment would involve a multi-faceted approach in partnership with my patients. I would explain the urgency and the systemic risks to my patients. I would offer salivary diagnostics to identify the pathogens present in their biofilm and provide treatments aimed at those pathogens. I would utilize all of the technology available to me to repeatedly disrupt the biofilm and attempt to create a stable, healthy microbiome.
I would partner with them to find additional devices that would assist them in biofilm disruption. We would seek out products to bolster their innate immunity with the goal of rendering them less susceptible to the bacterial challenge. All patients with active inflammation were scheduled for two-, three- or four-month recare appointments.
Why Are Protocols Slow to Change?
Even now, nine years after the Hajishengallis study was published, I still find that the majority of dental hygienists seem to base their practice protocols on the old model of periodontal disease. They delay the treatment of periodontal disease until advanced stages. Rarely do I encounter hygienists that intervene at the earliest stages of disease, and I believe that a combination of factors is the cause.
Coaching clients, dentists, and dental hygienists have admitted that rather than upsetting patients by telling them they have periodontal disease, they perform prophylaxis procedures regardless of the patient’s condition. While I try not to be judgmental, this behavior is obviously unethical, unprofessional, and unacceptable.
In addition, not every dentist or hygienist has the inclination to read research and choose continuing education courses based on the latest science, so they simply may not know. I remember being a very young hygienist with young children and having a huge backlog of journals on my desk, so I understand that it can be tough to keep up.
However, staying current is part of being a health care professional, and now, more than ever, there are abundant programs and presenters who provide this education. I teach all of my client teams the science of the oral-systemic links and how to easily and efficiently integrate that science into practical clinical protocols. Patients are becoming more aware of the links between oral disease and systemic diseases thanks to mainstream media, and they expect us to discuss their risks with them. Communication skills to facilitate these conversations and increase treatment acceptance are also part of my coaching.
Understanding the polymicrobial synergy and dysbiosis model will enable clinicians to develop more proactive and effective therapeutic approaches in treating periodontal and systemic diseases and preventing disease recurrence. By taking better care of our patients, we grow our practices with maximized revenue, optimized patient retention, and increased new patient referrals.
Need CE? Click Here to Check Out the Self-Study CE Courses from Today’s RDH!
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Teles, R. Teles, F. et. al. Lessons learned and unlearned in periodontal microbiology. Periodontal 2000. 2013 Jun; 62(1):95-162. Published online 2013 April 11. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3912758/ Doi: 10,111/prd.12010
- Loesche, W.J., Grossman, N.S. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001; 14(4): 727-752. doi:10.1128/CMR.14.4.727-752.2001. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC89001/
- Hajishengallis, G. Lamont, R. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Microbiol Oral Immunol. 2012; 27: 409-419. Retrieved from https://doi.org/10.1111/j.2041-1014.2012.00663.x
- How, K.Y., Song, K.P., Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol. 2016; 7: 53. doi:10.3389/fmicb.2016.00053. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4746253/
- Hajishengallis, G., Diaz, P.I. Porphyromonas gingivalis: Immune subversion activities and role in periodontal dysbiosis. Curr Oral Health Rep. 2020; 7(1): 12-21. doi:10.1007/s40496-020-00249-3. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7747940/
- Giannobile, W.V. Salivary diagnostics for periodontal diseases. J Am Dent Assoc. 2012 Oct; 143(10 Suppl): 6S-11S. doi: 10.14219/jada.archive.2012.0341. PMID: 23024320. Retrieved from https://pubmed.ncbi.nlm.nih.gov/23024320/
- Li, X., Kolltveit, K.M., Tronstad, L., Olsen, I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000; 13(4): 547-558. doi:10.1128/cmr.13.4.547-558.2000. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC88948/