With the increased controversy of fluoride obscuring large amounts of patients’ opinions, it is imperative that dental hygiene professionals are educated on fluoride alternatives so they may guide their patients accordingly. While there are several options for those wishing to remain fluoride-free, I find the hydroxyapatite (HAP) studies compelling and superior to other alternatives.
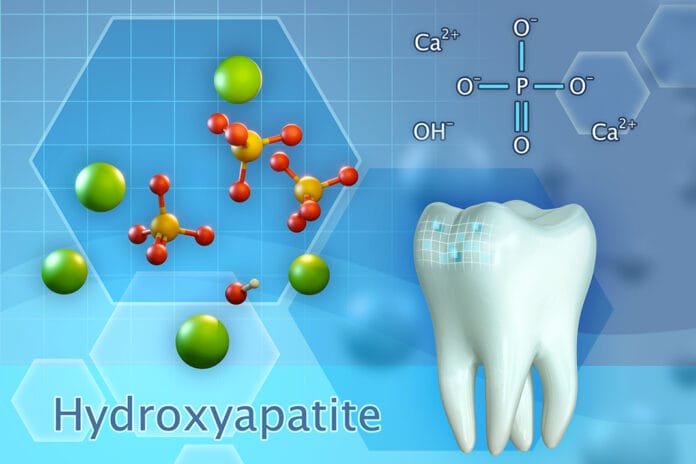
Hydroxyapatite
First, let’s remind ourselves of the history of this enthralling element. The hardest substance in the human body is none other than tooth enamel. Enamel is the outermost layer of the human tooth and is comprised of mostly hydroxyapatite, a hexagonal crystalline structure with a particle size of 5 nm to 20 nm.1 However, enamel also contains 4% organic material: magnesium, sodium, fluoride, carbonate, and water.2
Enamel is the outer protective layer for the softer layers of the tooth, dentin and pulp, making this organic material vital to the strength of the enamel.1 Hydroxyapatite is the bioavailable form of calcium used by our bodies to rigidify and strengthen the bone and the hard structures of our teeth – it is also found in saliva.2
Nano-hydroxyapatite (nano-HA) is one of the most popular synthetic variables of this organic resource. Nano-HA was first obtained from the brains of NASA. While in space, astronauts lose minerals that support the bones and teeth due to the absence of gravity. To remedy this problem, NASA proposed incorporating brushite (calcium, hydrogen, phosphate, and water) into a gel solution.1
Thus, in 1974, nano-hydroxyapatite was born. The rights were then sold to Sangi Co., LTD, a Japanese company, in 1978, which then integrated nano-HA into a toothpaste for the purpose of enamel remineralization.1
Synthetic hydroxyapatite is highly biocompatible and has a close analogy to the inorganic structure of tooth enamel. This variable presents as a lattice-like structure and has a chemical formulation of calcium hydroxyapatite [Ca10(PO4)6(OH)2].3
Depending on the manufacturing technique, various synthetic apatites are being produced. Synthetic HAP used in dentistry is tailored to achieve properties such as crystal morphology (spherical or needle-like), crystal size (micro vs. nano), and crystal purity. Studies report that clinical effectiveness is dependent upon specific geometry, pore size, and degradation rate of the HAP, making a smaller particle size more desirable.3
Nano-HA vs. Micro-HA
In nano-HA, rod-shaped nanocrystalline particles resembling those in natural enamel have a size ranging from 20 nm to 100 nm. Due to an increased surface area, nano-HA has the ability to bind to surfaces better, thus increasing the effectiveness of remineralization and reduction in tooth sensitivity as this particle size matches that of acid erosion at the enamel surface. Nano-HA particles are small enough to react with the bacterial membrane as the particle size is smaller than the actual microorganisms, making them more effective in biofilm management in the oral biome than their counter partner, microcluster hydroxyapatite.3
Microcluster hydroxyapatite, also known as micro-HA, has a particle size of five to 10 microns, significantly larger than organic enamel HAP or dentinal tubules. Due to this factor alone, micro-HA is not as effective as nano-HA when used in dentistry.3
Micro-HA is a calcium compound containing minerals in their natural ratios, derived from whole bone. Micro-HA powder is synthesized from calcium acetate and triethyl phosphate in a water and ethanol medium.5 While not as effective as nano-HA, this variation still provides benefits in dentistry among oral care products such as biofilm management and tooth whitening.6
Reviewing Hydroxyapatite Benefits
A debate among dental professionals is the concentration of hydroxyapatite necessary to achieve maximum benefits. Multiple studies provide different results for various concentrations. It is advised that a 10% HAP may be optimal for tooth remineralization, while another study suggests a 15% HAP concentration as being comparable to highly concentrated prescription-based fluoride of 12,500 ppm.9,10 However, it has been determined that a 15% HAP is too high for practical use in mouth rinse and toothpaste as it does create some levels of aggregation.11
While the main focus for hydroxyapatite seems to be enamel remineralization, this substance has also proven to provide other benefits. In 1987, the first clinical trial on HAP toothpastes against sensitivity was conducted. This trial determined that HAP toothpaste relieved dental hypersensitivity better than local benzocaine anesthetic used in other toothpastes.6
Further studies concluded that the nano-HA derivative worked best. Hydroxyapatite works by binding to and coating the surface, as well as occluding the dentin tubules.8 A 2023 Biomimetics article noted, “Specific crystal morphology and size have not been studied in detail to determine what the optimum dimensions for biomimetic HAP should be for dentin tubule occlusion and dentin adherence.”8
A total of 48 studies have been published corroborating the effectiveness of HAP in reducing dental sensitivity.8
Hydroxyapatite is also successful as a 0.2% chlorhexidine rinse in reducing biofilm.7 Both in situ and in vitro studies using subminimal concentrations show inhibitory effects, meaning it does not kill bacteria in the process. Further studies prove that HAP is also effective in reducing bacterial attachment to suture threads.7
Hydroxyapatite has even been studied and clinically tested as an at-home tooth whitener. When incorporated into toothpaste, HAP has been shown to help lighten enamel. Biometric HAP is made up of opaque white particles that, when deposited, help mask the yellow hue of enamel and also assist in removing extrinsic stains.8 HAP can provide an immediate result when the micro-crystals of HAP fill micro-defects on the enamel’s surface, remineralizing the lost apatite from the surface and providing long-term results when used daily.8 A 2021 Materials article noted, “However, the whitening degree may only be equal to 50% of the whitening effect of bleaches like hydrogen peroxide.”3
Fluoride has been a long-standing contributor to caries reduction and remains imperative in oral health as it is superior to other agents in tooth remineralization. Currently, the American Dental Association Council on Scientific Affairs and the Center for Evidence-Based Dentistry does not recommend using HAP over fluoride for enamel remineralization.12 However, the compelling research on synthetic hydroxyapatite opens doors for dental professionals when presented with patients looking for fluoride alternatives. While I firmly believe educating patients on factual research on fluoride is significant, I also try to respect their beliefs by simply providing them with effective alternatives.
Before you leave, check out the Today’s RDH self-study CE courses. All courses are peer-reviewed and non-sponsored to focus solely on high-quality education. Click here now.
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Pepla, E., Besharat, L.K., Palaia, G., et al. Nano-hydroxyapatite and its Applications in Preventive, Restorative and Regenerative Dentistry: A Review of Literature. Annali di Stomatologia. 2014; 5(3): 108-114. http://eprints.bice.rm.cnr.it/11504/1/article.pdf
- Byrum, K. Nano-hydroxyapatite vs. Fluoride. American Student Dental Association: Contour. 2020; 4(4): 34-35. http://digitaleditions.walsworthprintgroup.com/publication/?m=44499&i=654899&p=36&ver=html5
- Chen, L., Al-Bayatee, S., Khurshid, Z., et al. Hydroxyapatite in Oral Care Products – A Review. Materials. 2021; 14(17): 4865. https://doi.org/10.3390/ma14174865
- Kuśnieruk, S., Wojnarowicz, J., Chodara, A., et al. Influence of Hydrothermal Synthesis Parameters on the Properties of Hydroxyapatite Nanoparticles. Beilstein Journal of Nanotechnology. 2016; 7: 1586-1601. https://doi.org/10.3762/bjnano.7.153
- Vijayalakshmi, U., Rajeswari, S.S. Preparation and Characterization of Microcrystalline Hydroxyapatite Using Sol Gel Method. Trends in Biomaterials and Artificial Organs. 2006; 19(2). https://www.researchgate.net/publication/45626313_Preparation_and_Characterization_of_Microcrystalline_Hydroxyapatite_Using_Sol_Gel_Method
- Meyer, F., Enax, J., Amaechi, B.T., et al. Hydroxyapatite as Remineralization Agent for Children’s Dental Care. Frontiers in Dental Medicine. 2022; 3. https://www.frontiersin.org/articles/10.3389/fdmed.2022.859560/full
- Meyer, F., Enax, J. Hydroxyapatite in Oral Biofilm Management. European Journal of Dentistry. 2019; 13(2): 287-290. https://pubmed.ncbi.nlm.nih.gov/31574542/
- Limeback, H., Enax, J., Meyer, F. Clinical Evidence of Biomimetic Hydroxyapatite in Oral Care Products for Reducing Dentin Hypersensitivity: An Updated Systematic Review and Meta-Analysis. Biomimetics. 2023; 8(1): 23. https://doi.org/10.3390/biomimetics8010023
- Amaechi, B.T., AbdulAzees, P.A., Okoye, L.O., et al. Comparison of Hydroxyapatite and Fluoride Oral Care Gels for Remineralization of Initial Caries: A pH-cycling Study. BDJ open. 2020; 6: 9. https://doi.org/10.1038/s41405-020-0037-5
- O’Hagan-Wong, K., Enax, J., Meyer, F., Ganss, B. The Use of Hydroxyapatite Toothpaste to Prevent Dental Caries. Odontology. 2022; 110(2): 223-230. https://doi.org/10.1007/s10266-021-00675-4
- Anil, A., Ibraheem, W.I., Meshni, A.A., et al. Nano-Hydroxyapatite (nHAp) in the Remineralization of Early Dental Caries: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(9): 5629. https://doi.org/10.3390/ijerph19095629
- Slayton, R.L., Urquhart, O., Araujo, M.W.B., et al. Evidence-based Clinical Practice Guideline on Nonrestorative Treatments for Carious Lesions. Journal of the American Dental Association. 2018; 149(10): 837-849.e19. https://jada.ada.org/article/S0002-8177(18)30469-0/fulltext