In 1998, Dr. Sigmund Socransky developed the “complex theory” where periodontal pathogens are categorized based on their association with the severity of disease. In the complex theory, periodontal pathogens have been identified and classified by color to indicate which bacteria are associated with the onset and progression of periodontal disease. The classification includes the red complex, orange complex, green complex, orange-associated complex, and an Aa complex.
Bacteria in the green and orange-associated complexes are considered early colonizers. They adhere to the pellicle and are necessary for the colonization of other bacteria associated with periodontal disease.
The orange complex consists of bacteria that enable crosstalk between bacteria often referred to as the “bridging species.” Think of them as a bridge between early colonizers and the more pathogenic bacteria that we find in the red complex. Orange complex bacteria are also associated with increased pocket depth and progressive attachment loss.
The red complex and Aa complex are the final bacteria that colonize and lead to the destruction of the periodontium. Without the previously mentioned bacteria in the green, orange-associated, and orange complexes, the red complex bacteria are rarely able to colonize.1

Socransky’s Criteria
In 1890, Robert Koch developed a list of criteria to determine whether a specific bacterium was the cause of a given disease. Today we refer to that criterion as Koch’s postulates. The criteria to determine a true pathogen according to Koch’s postulates includes:
- The microorganism must be found in abundance in all organisms suffering from the disease but should not be found in healthy organisms.
- The microorganism must be isolated from a diseased organism and grown in pure culture.
- The cultured microorganism should cause disease when introduced into a healthy organism.
- The microorganism must be isolated again from the inoculated, diseased experimental host and identified as being identical to the original specific causative agent.
After reading through the criteria, it should be quite obvious that periodontal pathogens are not true pathogens, according to Koch’s postulates. Therefore, Socransky proposed a modified version to differentiate between commensal and opportunistic bacteria associated with periodontal disease. Socransky’s criteria include:
- A pathogen should be found more frequently and in higher numbers in disease states than in healthy states.
- Elimination of the pathogen should be accompanied by elimination or remission of the disease.
- There should be evidence of host response to a specific pathogen that is causing tissue damage.
- Properties of a putative pathogen that may function to damage the host tissues should be demonstrated.
- The ability of a putative pathogen to function in producing disease should be demonstrated in an animal model.
At the time, Socransky’s criteria were cutting edge and acknowledged that some of the bacteria − previously thought to be pathogenic − were commensal in certain hosts. In addition, this criterion brought to light the host response, which was not addressed in Koch’s postulates.
What We Learned from Complex Theory
Since the implementation of the complex theory and Socransky’s criteria, we have learned even more about periodontal disease. Many of the criteria put forth by Socransky have been challenged in recent years. Many question the use of the word “pathogen” in the criteria he proposed.
Challenges and questions concerning Socransky’s criteria point out that periodontal bacteria are capable of colonizing and proliferating in only the sites that provide the proper nutritional needs and metabolic requirements. Additionally, the bacteria found in the red complex has been found in individuals with a healthy periodontium, indicating that these bacteria are also commensal.2,3
Socransky’s criteria on elimination have been brought into question as many believe that there is no periodontal treatment that can effectively eliminate specific bacteria from the periodontal pocket. This is due to the fact that many are indigenous commensals.
Eliminating all the bacteria would not be beneficial as we now know the oral microbiome is a delicate balance. An imbalance in the oral microbiome can lead to whole-body systemic diseases. The elimination of certain bacteria could cause a shift from homeostasis to dysbiosis.
When considering host response and periodontal pathogens, as mentioned above, there is strong evidence that many of the periodontal pathogens identified in the complex theory are commensal. They only appear to cause disease when a change occurs in the host that allows the bacteria to flourish, resulting in dysbiosis of the oral microbiome and leading to periodontal disease.
The reason is currently unknown, but it has been postulated that the commensal bacteria become pathogenic due to alterations in the host. These alterations include inflammatory conditions and immune responses that make a more favorable environment for the bacteria to proliferate, leading to increased risk of disease onset and progression.
Lastly, animal studies have proven to be ineffective at predicting responses in humans. Although scientists can induce periodontal disease in animal models, it has proven to be very difficult to recover and culture the bacteria. This is not isolated to periodontal disease; many diseases are studied in animals that unfortunately cannot be extrapolated to humans.3
Polymicrobial Synergy and Dysbiosis Model
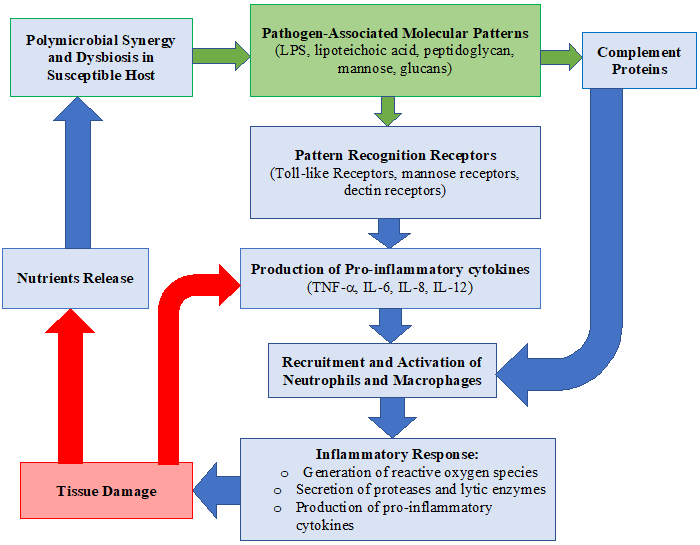
In 2012, the polymicrobial synergy and dysbiosis model of periodontal disease pathogenesis questioned the importance of the red complex. This model addresses the evidence that some pathogens, such as P. gingivalis, are incapable of pathogenicity alone. These pathogens require a community to become pathogenic.
The polymicrobial synergy and dysbiosis model suggests there are three fundamental prerequisites for periodontal pathogens to emerge as pathogenic:
- In the formation of a diverse community, microbial components will exhibit the appropriate receptors and adhesions.
- Each representative of the community will be co-operative and harmonious
- The community as a whole will withstand the inherent as well as attained host immunity and add to inflammation of the periodontal tissues.
Ultimately, this model shows that subgingival plaque cultured from a healthy periodontal pocket has the same potential as subgingival plaque found in diseased sites to produce inflammation. However, the host immune response must be diverted by keystone pathogens, and colonization is then achieved by accessory pathogens and over-activated by pathobionts. Therefore, the pathogenesis of periodontal disease is based on polymicrobial synergy and dysbiosis.
This concept has been applied to other chronic diseases such as inflammatory bowel disease and colon cancer. These chronic diseases, much like periodontal disease, have shown no single or select few species are associated with the onset and progression of disease, but rather microbial communities cause pathology.4,5
New Emerging Periodontal Pathogens
Recently two Gram-positive anaerobic bacteria have emerged as important periodontal pathogens, Filifactor alocis, and Peptoanaerobacter stomatis. Both of these pathogens manipulate neutrophil effector functions.
F. alocis has been shown to withstand neutrophils’ antimicrobial response. The mechanism by which the pathogen evades the neutrophils’ antimicrobial response is its ability to inhibit complement activation and prevent intracellular reactive oxygen species (ROS) production. Therefore, even if the bacteria are engulfed by the neutrophil it is not destroyed through phagocytosis.
P. stomatis also has the ability to evade phagocytosis by neutrophils while promoting cell activation. It has been shown to degranulate neutrophil granules helping to maintain high levels of ROS which could contribute to damaging host tissue and propagating the inflammatory response.6,7,8
Conclusion
Although I did not include every hypothesis or model proposed since Socransky presented the complex theory, it is evident that, as we learn more about the human body, we learn more about certain chronic diseases such as periodontal disease. At the time, Socransky’s complex theory was cutting edge and quite impressive.
Since then we have discovered that bacteria are required, but not sufficient enough alone to initiate the onset of periodontal disease. Additionally, a few novel pathogens that play a role in the onset and disease progression have since been discovered. We have also learned host susceptibility is a part of the bigger picture. Therefore, we must go beyond brushing and flossing instructions and incorporate the patient’s overall health to guide the way we counsel our patients.
This is an example of why it is imperative to continue to update our knowledge as new research emerges. Science is ever-evolving as we learn more, we must implement evidence-based practices that reflect the most current research guiding our patients to better oral health.
Now Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Mohanty, R., Asopa, S.J,, Joseph, M.D,, et al. Red complex: Polymicrobial conglomerate in oral flora: A review. J Family Med Prim Care. 2019; 8(11): 3480-3486. Published 2019 Nov 15. doi:10.4103/jfmpc.jfmpc_759_19. Retrieved from http://www.jfmpc.com/article.asp?issn=2249-4863;year=2019;volume=8;issue=11;spage=3480;epage=3486;aulast=Mohanty.
- Bartold, P.M., Van Dyke, T.E. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000. 2013; 62(1): 203-217. doi:10.1111/j.1600-0757.2012.00450.x. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3692012/.
- Alwaeli, A.Z.J. (August 1, 2018). Anaerobic Bacteria Associated with Periodontitis, Oral Microbiology in Periodontitis, Sonia Bhonchal Bhardwaj, IntechOpen, DOI: 10.5772/intechopen.76352. Retrieved from https://www.intechopen.com/books/oral-microbiology-in-periodontitis/anaerobic-bacteria-associated-with-periodontitis.
- Shaikh, H.F.M., Patil, S.H., Pangam, T.S., Rathod, K.V. Polymicrobial synergy and dysbiosis: An overview. J Indian Soc Periodontol. 2018; 22(2): 101-106. doi:10.4103/jisp.jisp_385_17. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5939015/.
- Lamont, R.J., Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015; 21(3): 172-183. doi:10.1016/j.molmed.2014.11.004. Retrieved from https://pubmed.ncbi.nlm.nih.gov/25498392/.
- Vashishta, A., Jimenez-Flores, E., Klaes, C.K., et al. Putative Periodontal Pathogens, Filifactor Alocisand Peptoanaerobacter Stomatis, Induce Differential Cytokine and Chemokine Production by Human Neutrophils. Pathogens. 2019; 8(2): 59. Published 2019 May 1. doi:10.3390/pathogens8020059. Retrieved from https://pubmed.ncbi.nlm.nih.gov/31052371/.
- Edmisson, J.S., Tian, S., Armstrong, C.L., et al. Filifactor alocis modulates human neutrophil antimicrobial functional responses. Cell Microbiol. 2018; 20(6): e12829. doi:10.1111/cmi.12829. Retrieved from https://pubmed.ncbi.nlm.nih.gov/29377528/.
- Jimenez Flores, E., Tian, S., Sizova, M., Epstein, S.S., Lamont, R.J., Uriarte, S.M. Peptoanaerobacter stomatis Primes Human Neutrophils and Induces Granule Exocytosis. Infect Immun. 2017; 85(7): e01043-16. Published 2017 Jun 20. doi:10.1128/IAI.01043-16. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5478963/.