Patients often inquire about why I take vital signs at their dental appointments. I respond that the mouth is connected to the rest of the body and every type of health care provider should take vital signs at every visit.
Though both statements are true, it doesn’t really give the dental patient the real answer. As it relates to oral health, high blood pressure may increase the risk of periodontal disease. We may see patients with high blood pressure who have no clinical signs of periodontal disease. However, this does not mean they are not already in the early stages of the disease process.
It is unclear when the biological onset of periodontal disease begins. As a result, we often rely on clinical manifestations to diagnose and identify the disease. What if high blood pressure was considered a clinical manifestation? Should we use that as a factor when educating our patients on periodontal disease? Should the patient’s hypertensive status be considered when making periodontal prevention and maintenance treatment plans? Let’s explore these questions.
What are Foam Cells?
Cells are necessary for proper biologic function in the human body. However, cell types vary, and some cells can contribute to disease onset and progression. Though disease onset and progression can be triggered by multiple cell types, an interesting character in the cast of cells in the human body that not only contributes to disease but is triggered by periodontal pathogens is the foam cell.
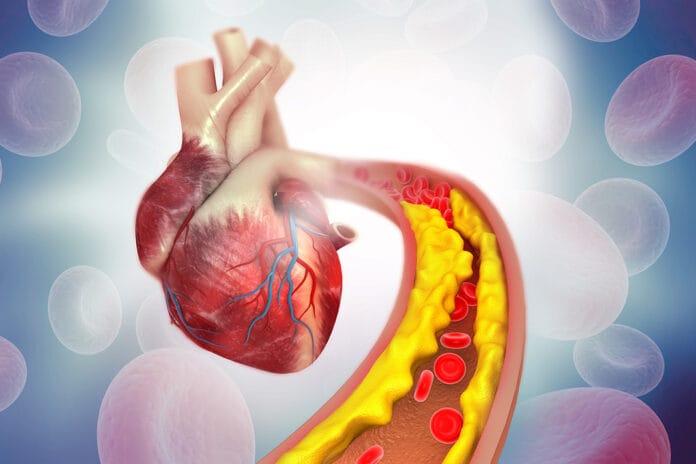
Foam cells are lipid-laden macrophages integral to all stages of atherosclerosis – from the formation of the initial fatty streak to the progression and pathogenicity of established plaques. Recognized since the mid-nineteenth century, their role in the inflammatory process leading to atherosclerosis has only recently been characterized.
As such, they are considered potential therapeutic targets for atherosclerosis treatment. Beyond atherosclerosis, foam cells have been identified in other forms of chronic inflammation, including septic arthritic lesions, periodontal disease, and tissues infected by persistent pathogens.1
Foam cell formation can impair macrophage immune function, contributing to disease pathogenesis. For instance, they play a crucial role in atherosclerosis, tuberculosis, multiple sclerosis, and possibly vaping-related lung injuries. The current understanding of foam cell formation is largely based on atherosclerosis studies, associating it with dysregulated cholesterol metabolism. However, recent research on tuberculosis suggests that foam cell formation may be disease-specific since these cells are enriched in triglycerides rather than cholesterol derivatives.2
Periodontal Pathogens Promote Foam Cell Formation
Periodontal pathogens, including Porphyromonas gingivalis, Fusobacterium nucleatum, Treponema denticola, and Tanenerella forsythia have been found in atherosclerotic plaques removed from patients’ arteries. This suggests that these bacteria could directly or indirectly contribute to the development of atherosclerotic lesions, potentially through the induction of inflammatory mediators.3
Atherosclerosis is a complex process involving many factors. It begins with the formation of foam cells. These foam cells are a major component of atherosclerotic plaques. Previous research has shown that periodontal pathogens can promote the accumulation of lipids in cells, leading to the formation of foam cells. However, the exact mechanisms and molecules involved are not yet fully understood.3
In this context, the role of cholesterol is crucial. When cholesterol is not efficiently excreted from cells and accumulates, it is stored as lipid droplets, contributing to the formation of foam cells. Research has shown that periodontal pathogens disrupt this lipid balance, leading to an accumulation of cholesterol in macrophages. This suggests that periodontal pathogens could contribute to the formation of foam cells and, therefore, atherosclerotic plaques and hypertension.3
The formation of foam cells involves the uptake of lipoproteins, the hydrolysis of cholesterol esters, and the re-esterification of cytosolic cholesterol. Research suggests that periodontal pathogens disrupt this process by modulating cholesterol influx and efflux-related enzymes rather than by increasing cholesterol esterification. This disruption of lipid homeostasis in macrophages could be a significant factor in the formation of foam cells. This is a proposed mechanism of action by which periodontal pathogens contribute to atherosclerosis and hypertension.3
Immune Evasion of P. gingivalis As a Contributing Factor in the Formation of Foam Cells
P. gingivalis expresses a variety of virulence factors, such as lipopolysaccharide (LPS), gingipains, fimbriae/pili, collagenase, (erythrocyte) lectins, capsule, protease, and superoxide dismutase that stimulate host immune responses and play an important role in promoting atherosclerosis progression. It evades host innate and adaptive immunity, internalizes in host tissues, and circulates with blood and lymph, colonizing arterial vessel walls and causing local inflammation and lesions.4,5
P. gingivalis interferes with the function of the complement system, a major part of the innate immune system. P. gingivalis also inhibits the antimicrobial function of immune cells, evading host immune killing, surviving in the host for a long time, repeatedly stimulating the body’s immune system, leading to a persistent low-level inflammatory state in the host, and promoting the progression of atherosclerosis and hypertension.4
The immune evasive abilities of P. gingivalis include evasion of death via neutrophils, and due to its ability to cycle in and out of cells, it can evade death via macrophages. This immune evasion leads to chronic inflammation and the formation of foam cells. This is another mechanism by which periodontal pathogens can induce the formation of foam cells leading to the onset and progression of atherosclerosis and hypertension.2,4
Conclusion
In conclusion, research suggests that periodontal pathogens play a significant role in the formation of foam cells, a key factor in the development of atherosclerosis and hypertension. This provides further evidence for the connection between periodontal disease and systemic diseases.
These findings also highlight the importance of taking vital signs at dental hygiene appointments. Additionally, it would be reasonable to use the patient’s hypertension status as a risk factor for periodontal disease when developing their periodontal prevention or maintenance plan.
Our understanding of the oral-systemic link continues to evolve and improve, allowing a better understanding of the connections. As dental hygienists, understanding this connection can help us educate our patients about the importance of maintaining good oral health, not just for their teeth but for their overall health as well.
Before you leave, check out the Today’s RDH self-study CE courses. All courses are peer-reviewed and non-sponsored to focus solely on pure education. Click here now.
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Valledor, A.F., Lloberas, J. and Celada, A. (2015, February 16). Macrophage Foam Cells. Wiley Online Library. https://doi.org/10.1002/9780470015902.a0020730.pub2
- Guerrini, V., Gennaro, M.L. Foam Cells: One Size Doesn’t Fit All.Trends in Immunology.2019; 40(12): 1163-1179. https://doi.org/10.1016/j.it.2019.10.002
- Rho, J.H., Kim, H.J., Joo, J.Y., et al. Periodontal Pathogens Promote Foam Cell Formation by Blocking Lipid Efflux.Journal of Dental Research. 2021;100(12): 1367-1377. https://doi.org/10.1177/00220345211008811
- Ruan, Q., Guan, P., Qi, W., et al. Porphyromonas gingivalisRegulates Atherosclerosis through an Immune Pathway.Frontiers in Immunology. 2023; 14: 1103592. https://doi.org/10.3389/fimmu.2023.1103592
- Jia, L., Han, N., Du, J., et al. Pathogenesis of Important Virulence Factors ofPorphyromonas gingivalisvia Toll-Like Receptors. Frontiers in Cellular and Infection Microbiology. 2019; 9: 262. https://doi.org/10.3389/fcimb.2019.00262