Medications cure, treat, or prevent a disease or condition or relieve symptoms from an illness ─ vital elements to maintaining or improving health. Over 20,000 prescription medications are approved for marketing, and more than 66% of all adults in the United States use prescription medications. 1
Dental patients are more often on medications than not. Dental professionals should evaluate the effects of medicines when considering possible oral or systemic health issues. A review of specific dental concerns follows the discussion of medications below.
Routes of Transmission
Medication has a variety of routes of transmission.2
Oral route: The most common and convenient. The medication starts with absorption in the mouth and stomach. Most medications are absorbed through the small intestine and pass to the liver and then transported via the bloodstream and to the targeted site. When taken orally, food and other medications may affect how much or how fast it’s absorbed through the digestive tract. Some medications are absorbed poorly in the digestive tract or are destroyed by acid and digestive enzymes.3
Injection route: Through the skin, this can be by needle, patches, or implantation.
Subcutaneous route: A needle is inserted into fatty tissue beneath the skin. The medication moves into the small blood vessels and then is transported through the bloodstream. Alternatively, some medications reach the bloodstream through the lymphatic vessels. Many protein medications are administered this way, so the digestive tract doesn’t destroy them.3
Implantation route: The device is inserted beneath the surface of the skin in the loose interstitial tissue of the upper arm, anterior surface of the thigh, or the lower portion of the abdomen. This route is rarely used, but it is worthwhile in providing a long-term therapeutic effect.3
Intramuscular route: An injection into a muscle is recommended when larger volumes of medication are needed. Since the muscle lies deeper below the skin and fatty tissue, a longer needle is needed. This injection is commonly in the upper arm, thigh, or buttock. How quickly it is absorbed into the bloodstream depends on the blood supply to the muscle. The sparser the blood supply, the slower the absorption, and vice versa ─ the more abundance the blood supply, the faster the absorption.3
Intravenous route: The needle is inserted directly into the vein, either a single dose or continuous infusion. Its inserted through a collapsible plastic bag, infusion pump, or catheter. This is the most effective way to deliver a precise, well-controlled dose quickly. It is also used for irritating solutions, which may cause pain and tissue damage if given via the intramuscular or subcutaneous routes.3
Intrathecal route: A needle is inserted between two vertebrae in the lower spine and the space around the spinal cord. This is used when a drug is needed to produce rapid or local effects on the brain, spinal cord, or the meninges.3
Sublingual and buccal route: The medication is placed under the tongue or between the teeth and gingiva and is absorbed quickly. It immediately goes into the bloodstream without first having to pass through the intestinal wall and liver.3
Rectal route: Many medications administered orally can also be administered rectally. In this form, it is coated in a waxy substance that dissolves or liquefies quickly as this region of the body’s lining is thin and rich in blood supply, causing rapid absorption.3
Vaginal route: This is usually with estrogen during menopause as a solution, cream, gel, suppository, ring, or tablet.3
Ocular route: Used to treat eye disorders, the medication is in a liquid, gel, or ointment form.3
Otic route: Administered with drops with a localized application to treat ear inflammation or infection.3
Nasal route: The medication is breathed in and absorbed through the thin mucus membrane lining of the nasal walls. Once absorbed from the tiny droplets, it quickly enters the bloodstream.3
Inhalation route: Small droplets are inhaled to purposely pass through the trachea into the lungs and then to the bloodstream. The smaller the droplets, the deeper into the lungs it advances and increases the amount of medication absorbed. Few medications are administered this way since breathing in the therapeutic amount within a specified time can be inconsistent.3
Nebulization route: Similar to the inhalation route, although devices are used such as an ultrasonic or jet nebulizer system. This technique is used to properly help maximize the amount of medication delivered to the lungs. The aerosols are transformed into small particles to reach the lungs.3
Cutaneous route: Directly applied to the skin for a localized effect, this route is used on skin disorders and infections.3
Transdermal route: A patch is placed on the skin to deliver systemically. It delivers slowly and continuously for many hours or days; it is used with medications that are quickly eliminated from the body.3
How Medications Work in the Body
Absorption: When the medication passes from the site of administration into circulation in the bloodstream, the blood carries the medication to the organs, especially the ones on which the medication acts on.4
Distribution: This is influenced by how well each organ is supplied by the blood, organ size, binding of the medication, various tissues and blood components, and the permeability of tissue membranes—the more fat-soluble, the greater its ability to cross through the membrane. Protein binding is important as many medications bind to blood proteins to be active.4
Metabolism: Occurs by changing the active part of the medications and initiating them to be more water-soluble and ready for excretion by the kidneys, as the body works to remove medication perceived as a foreign agent.
This reaction involves different processes. Oxidation and reduction processes enforce the charge of the molecule, either more negative or more positive, making them dissolvable in water. These actions occur mainly in the liver by enzymes. Conjugation (adding another compound) is needed to form molecules by adding other molecules. These reactions inactivate the pharmacologic activity of the medication and make it ready for elimination by the kidneys.4
Excretion: This happens primarily through urine. Although when the medication is not absorbed from the intestines or has been secreted in the bile, it will be cleared through fecal matter. Other routes of secretion are the way of expired air through the lungs, perspiration, and breast milk.4
Categories of Medications
Prescription medications are categorized as controlled, noncontrolled, and nonprescription (over-the-counter). Controlled medications are narcotics or scheduled medications and have the potential for abuse or dependence. Noncontrolled medications are prescription medications that are not controlled. Nonprescription medications are purchased at a store without a prescription.5
Drug Scheduling
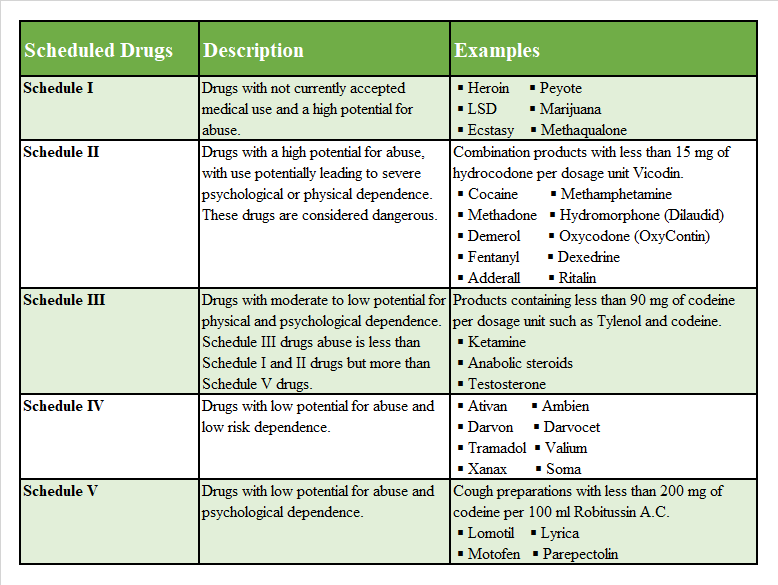
Drugs, substances, and certain chemicals are classified depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. Since 1970, the United States has maintained the Controlled Substance Act to protect the general public from potentially dangerous and addictive drugs. This is a way for the government to organize and categorize different drugs based on their potential to harm the general public. The abuse rate is a determinate factor in the scheduling of the drugs6 (see Figure 1).
Effects of Medications
Desired effect or therapeutic effect: The medication is doing its intended purpose.
Side effect: Almost all medications with a systemic effect will have side effects. Most side effects are not serious and decrease as the body becomes acclimated to the medication. For example, blood pressure medications can cause fatigue because of the way they work on the heart.
Adverse effect: This may be related to an increased dosage or accumulation of a medication to cause toxicity. Toxicity can cause damage to tissue and organs and even cause death.
Tolerance and dependence: Tolerance happens over time with repeated doses, and the individual’s response to the medication is decreased. Tolerance has occurred when the body has adapted to the minor side effects. Dependence is when physical or psychological development is needed for medication.
Interactions: This occurs between medications or medications and foods. Two or more medications can reduce or enhance a stronger response.
No apparent effect: The medication is not working and having no effect.
Paradoxical: Medications that work in the opposite way. For example, when pain is caused by a pain relief medication.5
FDA Approval
For consumer safety and protecting public health, the Food and Drug Administration (FDA) controls the drug-approval process and review new medications. The agency is part of the U.S. Department of Health and Human Services. The full research development and approval process can take 12 to 15 years and cost over 2.6 billion dollars. The FDA regulates the approval of medications; it doesn’t regulate the practice of medicine.7
According to the FDA website, a five-step process is completed for medications to be approved:
- Discovery and development: Discovery process is the discovering of a new drug by new insights into a disease, tests of molecular compounds for beneficial effects of a large number of diseases, existing treatments with unanticipated effects, new technologies to provide new ways to target medical products in specific sites within the body or to manipulate genetic material. The development process is to identify a promising compound by gathering information on absorption, distribution, metabolism, excretion, mechanism of action, dosage, route of transmission, side effects, adverse events, effects on different groups of people, interactions with medications and treatments, and effectiveness compared with similar medications.8
- Preclinical research: Testing the medication in vitro and in vivo. This is testing for safety, serious harm, and toxicity.8
- Clinical research: Testing, studies, and trials in humans.8
- FDA review: If proven, and the medication is effective and safe for its intended use, then the data, studies, analyses, and results are submitted for FDA review. The review team has 6- 10 months to decide on approval. Each technical disciplined team member evaluates their specialty from the clinical data. Inspectors travel to clinical sites for evidence of fabrication, manipulation, or withholding of data. The project manager assembles all individual reviews, documents, and inspection reports and issues a report to the FDA senior official to arrive at a decision.8
- FDA post-market safety monitoring: While clinical trials provide information on medication efficiency and safety, it’s still impossible to have complete information about safety at the time of approval. The true picture of the medication’s safety evolves over months and years of the life of the medication while in the marketplace. The information collected during the research stages and FDA reviews is added on the labels as a caution for dosage and usage, as well as other measures for more serious issues.8
After testing, the pharmaceutical company sends the FDA a new application which must include the drug test results and manufacturing information to demonstrate the company can properly manufacture the drug. The data includes information gathered during the animal studies and human clinical trials and the company’s proposed label for the drug, including uses for which it has been shown to be effective with possible risks and how to use the drug.7
Brand Name vs. Generic
The brand name is the more common and most familiar name for the medication and tends to be more expensive. This is the medication that takes years and millions of dollars to research, develop, be patented, and then approved by the FDA. Once this happens, the pharmaceutical company is the only company allowed to sell it for a specified amount of time. With this monopoly, the pharmaceutical company can charge any price they want. A patent on a medication currently lasts for 20 years.9
Generic drugs must also have FDA approval, although they don’t need to repeat the clinical trials of the brand name drug. The application for the generic drug is called the abbreviated new drug application (ANDA). Abbreviated refers to the drug companies not needing to include the animal and human data to establish safety and effectiveness. The ingredient in the drug must be as effective against the treated illness or condition treated as the brand name drug. It also must show the same amount of drug gets to the bloodstream in the same amount of time.7
Off-Label
Off-label is when an FDA-approved medication is used in a way that the FDA has not approved. It is a legal practice, and one in five prescriptions is written for off-label use. For example, when medication will be prescribed for off-label use, the medication is not approved to treat a certain condition or when a dose is different from what was approved. Treating patients with an off-label medication prescription can be really helpful with rare conditions, providing treatment or relief for symptoms unavailable with other treatments.9
In most cases, off-labels provide more benefit than risk. The risk of off-label use is the unknown side effects; it may not be fully effective for the prescribed use, and dosages may be incorrect.
Black Box Warning
Black box warning is the FDA’s most rigorous warning for drugs and medical devices on the market. Its purpose is to alert the public and health care providers of serious effects such as permanent side effects, injury, or death. The information is presented with a header in a box with all capitalized letters and information printed in bold typeface.
Drug companies are responsible for creating the information on a drug label, while only the FDA has the authority to issue a black box warning. If there is a black box warning, manufacturers must also create a medication guide that describes how patients can safely use the drug.10
According to its website, the reason the FDA would issue a black boxed warning is to highlight the risks:
- If a drug causes a severe adverse reaction such as potentially fatal life-threatening or permanently disabling where the risks might outweigh the benefits.
- A serious side effect can be avoided or reduced in severity or frequency by appropriate use of the drug, such as avoiding use in specific situations, observing patients, careful patient selection, or avoiding using the drug with certain medications.
- The drug is less effective or dangerous to certain populations such as the elderly, children, or pregnant women.
- The FDA approved the drug only for restricted use to ensure public safety.10
Blockbuster Drugs
Blockbuster drugs are medications that generate at least $1 billion in annual sales.
Pharmaceutical companies spend a gross amount of money on research and development and sell a successful drug at a high price to recoup losses and earn a profit. When a medication patent expires and generic medications flood the market, it negatively impacts sales. Once the generic versions are released, the price significantly reduces, erasing the monopoly and creating a competitive market. Common medications in this category include Advair, Humira, Lipitor, Vioxx, and Zoloft.11
In the United States, medication costs are unregulated, leaving the negation between the pharmaceutical and insurance companies. High prices contribute to the lack of ability for organizations to negotiate prices and giving power to the pharmaceutical companies.
Polypharmacy
Polypharmacy is the use of five or more medications taken daily. Individuals with one or more chronic conditions will have a longer medication list. This can initiate an increased risk in adverse drug reactions, drug interactions, prescribing cascade, and higher costs.
The more medications one takes, the more side effects are involved. There is an increase in more problems with not remembering why a medication is taken, when the medication should be taken, the timing of taking them, or altogether forgetting to take them.
Polypharmacy accounts for about 30% of all hospital admissions and is a top leading cause of death in the United States.12 Taking multiple medications can cause multiple side effects of which can be misdiagnosed or mistreated for real conditions.
Half-life
The half-life of a medication is how long it takes for half the dose to be metabolized and eliminated from the bloodstream. Medications with a short half-life become effective faster, but the downside is that it’s harder to come off it, creating a dependency. Since the medication needs to be taken more often, which can become addictive, it varies from person to person and is dependent on age, weight, genetics, and health issues.
A medication’s half-life is important when quitting the medication as it determines the duration and how the schedule is tapered off.13
Achieving a steady state is the end goal. This is the point when the medication amount is equal in the body to what has been eliminated. It takes about four times the amount of time for the concentration of the medication to reach a steady-state in the body.13
Discontinuation Syndrome
Several medications have discontinuation syndrome and need to be stopped in doses. If stopped abruptly or several doses are missed, withdrawal symptoms will occur. Discontinuation syndrome can be prevented with titration. Under physician care, gradually lowering the dose of a medication until it’s minimized or completely ceased is the safest way.
Depending on the medication type, it can take weeks or months. Antidepressants are the common medications that require this process.14
Common symptoms during discontinuation include:
- Dizziness, vertigo, or muscle coordination problems
- Paresthesia, numbness, electric shock sensations, tingling or pricking of the skin
- Lethargy, headache, tremor, sweating, anorexia
- Insomnia, nightmares, excessive dreaming
- Nausea, vomiting, diarrhea
- Irritability, anxiety, agitation, low mood14
Pregnancy Risk Category
Pregnancy risk categories changed in 2015 from the alphabet system to narrative sections and subsections. The old five-letter system seemed to provide vague information with misleading results. The FDA’s new labeling system, the Pregnancy and Lactation Labeling Rule (PLLR) allows better patient-specific counseling and informed decision-making for pregnant women.
This new process was gradually phased in, but it is still a little scattered. The labels on medications approved after June 30, 2001, will be phased in. Medications with labels approved prior to June 29, 2001, are not subject to the PLLR rule, although the letter category system has been removed from these medications since June 29, 2018. This also applies to generic medications. On the other hand, over-the-counter medications are not subject to the PLLR by the FDA.15
Pediatric Medication
The safety of dosages of medicine for children is important to calculate. Factors involved in medicating children involve height, weight dynamics of growth and maturation of organs, changes in metabolism with growth, changes in body proportion, and other developmental changes. The growth of the kidney and liver, where the medication is metabolized, is still in progress. Although many children maybe the size of a small adult, the dosing is still differential due to the underdevelopment and growth of essential body parts, organs, and brain.
Medication errors are common with children due to the dosage calculation of figuring out a smaller dosage from an adult dosage. Metabolism is also considered as children tend to have a faster one. A heavier child, for example, will need a higher dosage.16
Geriatric Medication
Medication intervention is important in the lives of the geriatric generation since they take more medications than the younger generations. Without medications, this group’s lifestyle will diminish with function or even dying prematurely. Chronic medical conditions may be worsened by medications or affect how they work.
Polypharmacy in this age group has a higher risk of drug interactions. Medication doses are more challenging to maintain as geriatrics are more sensitive to the effects of medications.
Medication risks are damage to the liver and kidney from years of use. Kidneys are less able to excrete medications into urine. The liver is less able to metabolize certain medications and excrete them from the body. With the older generation, water in the body decreases, causing medications that are water-soluble to reach higher concentrations because there is less water to dilute them. Fat tissue increases with age causing fat-soluble medications to accumulate and store more in fat tissue to cause toxicity.17
Obesity
Obesity has a significant impact on many organ systems that are associated with medication metabolism. The dosing is challenging with the effect of absorption, distribution, metabolism, and clearance.
A common problem with obesity is fatty liver disease ─ fat buildup in the liver causing scarring or inflammation ─ can cause permanent liver damage or failure. With ineffective critical organs, it may cause the effect of medications to be unpredictable.
In obesity, there is poor blood flow to fat, and more fat accumulation causes absorption and distribution of lipophilic medications to slowly take effect. In correlation with this, the excretion process is slower. The increased body fluid also increases the volume of distribution of water-soluble drugs. Due to more weight and slower effects on the body process, higher doses are necessary, which can increase issues of drug toxicity.18
Medications and Oral Health
Much of the information below was reported in the Journal of the American Dental Association or WebMD.
- Blood-thinning medications may lead to abnormal bleeding. They can cause excessive bleeding with dental surgery or cleanings. These medications include anticoagulants, aspirin, heparin, and warfarin.19
- Medications that alter taste (dysgeusia) can cause metallic, bitterness, or the inability to taste. These medications include cardiovascular agents, central nervous system stimulants, nonsteroidal anti-inflammatories, respiratory inhalants, and smoking-cessation products such as nicotine skin patches.19
- Some medications can cause reactions with soft-tissue and cause the development of oral sores, inflammation, discoloration of oral tissue. These medications include blood pressure, oral contraceptives, immunosuppressive agents, and chemotherapeutic agents.19
- Some medications can cause overgrown and enlarged tissue (gingival hyperplasia). These medications include antiseizure, immunosuppressants related to organ transplants, and calcium channel blockers.19
- Medications may cause xerostomia and reduce salivary flow. These medications include antihistamines, decongestants, painkillers, high blood pressure, muscle relaxants, antidepressants, urinary incontinence, and Parkinson’s.20
- Oral candidiasis or fungal infections can be caused by oral inhalers.20
- Tongue coating is a white, black, or yellow layered residue on the tongue. Medications that can cause tongue coating include antiviral, antibiotics, steroids, blood thinning, antipsychotic, and diabetes.19
- Some medications may lead to bruxism, the act of clenching or grinding the teeth. These medications include antipsychotics, antidepressants, and psychotropic agents.
- Medications that contain sugar may increase caries risk. These medications include liquid form, cough syrup and drops, vitamins, antacid tablets, and antifungal agents.19
- Mucositis is the inflammation of the moist tissue lining the mouth. Chemotherapy treatment is one example that may cause mucositis.20
- Certain medications may lead to internal stains (tooth discoloration of brown, yellow-brown, green, or gray). These medications include fluoride, tetracycline, minocycline, and ciprofloxacin.20
- Jaw necrosis is the progression of death of the jawbone. Medications that may cause jaw necrosis include anti-angiogenic and anti-resorptive agents, including Fosamax, other bisphosphonate agents, and Denosumab.19,20
More patients are on medications than not. Many even take multiple medications increasing side effects. Evaluating the whole picture of age, polypharmacy, and certain medications taken will prepare the dental professional for possible oral or systemic health issues.
Need CE? Click Here to Check Out the Self-Study CE Courses from Today’s RDH!
Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Prescription Drugs. (n.d.). Georgetown University: Health Policy Institute. Retrieved from https://hpi.georgetown.edu/rxdrugs/.
- (2011, March 20). Difference Between Drug and Medicine. Difference Between. Retrieved from https://www.differencebetween.com/difference-between-drug-and-vs-medicine/
- Le, J. (2020 October). Drug Administration. Merck Manual. Retrieved from https://www.merckmanuals.com/home/drugs/administration-and-kinetics-of-drugs/drug-administration
- How do Drugs Work? (n.d.). Editors Web. Retrieved from http://www.editorsweb.org/medications/drugs-work.htm
- What is Medication? (2011) BDS Medication Administration Curriculum Section II. Retrieved from https://www.dhhs.nh.gov/dcbcs/bds/nurses/documents/sectionII.pdf
- Drug Scheduling. (n.d.). United States Drug Enforcement Administration. Retrieved from https://www.dea.gov/drug-scheduling
- Miller, E. (2020, April 21). FDA Approval Process. Retrieved from https://www.drugwatch.com/fda/approval-process/#
- The Drug Development Process. (2018, January 01). S. Food & Drug Administration. Retrieved from https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process
- Billingsley, A. (2021, January 13). What Does ‘Off-Label’ Use Mean for Medications? Good Rx. Retrieved from https://www.goodrx.com/blog/off-label-drug-prescriptions/
- Llamas, M. (2020, April 13). Black Box Warnings. Retrieved from https://www.drugwatch.com/fda/black-box-warnings/
- Chen, J. (2020, June 10). Blockbuster Drug. Investopedia. Retrieved from https://www.investopedia.com/terms/b/blockbuster-drug.asp
- U.S Pharmacist. 2017; 42(6): 13-14. Retrieved from https://www.uspharmacist.com/article/polypharmacy
- Purse, M. (2019, September 29). Overview of Your Medication. Retrieved from https://www.verywellmind.com/medication-half-life-380031
- Grohol, J.M. (2016, May 17). What is Discontinuation Syndrome? Retrieved from https://psychcentral.com/lib/what-is-discontinuation-syndrome#1
- FDA Pregnancy Categories. (2021, February 2). com. Retrieved from https://www.drugs.com/pregnancy-categories.html
- Drug Research and Children. (2016, May 04). S. Food & Drug Administration. Retrieved from https://www.fda.gov/drugs/information-consumers-and-patients-drugs/drug-research-and-children
- Ruscin, J.M, Linnebur, S.A. (2018, December). Aging and Drugs. Merck Manual. Retrieved from https://www.merckmanuals.com/home/older-people%E2%80%99s-health-issues/aging-and-drugs/aging-and-drugs
- Yartsef, A. (2017, December 04). Influence of Morbid Obesity on Pharmacokinetics. Deranged Physiology. Retrieved from https://derangedphysiology.com/main/required-reading/pharmacology-and-toxicology/Chapter%201.2.2/influence-morbid-obesity-pharmacokinetics
- How Medications can Affect Your Oral Health. 2005; 136: 831. Retrieved from https://www.ada.org/~/media/ADA/Publications/Files/patient_51.pdf?la=en
- Oral Side Effects of Medications. (2020, August 11). Retrieved from https://www.webmd.com/oral-health/guide/oral-side-effects-of-medications#4