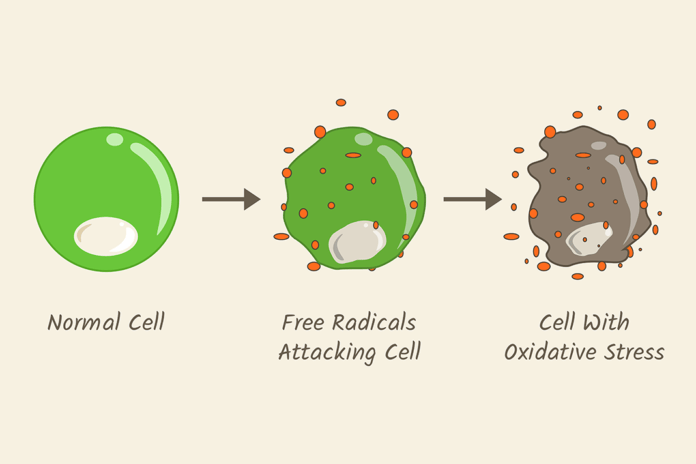
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS/free radicals) and antioxidants in the body. Prolonged oxidative stress is associated with a wide range of chronic illnesses, which includes, but is not limited to, cancer, diabetes, rheumatoid arthritis, nonalcoholic fatty liver disease, chronic inflammation, stroke, and numerous neurodegenerative diseases.1
As with most bodily functions, there is a little chemistry (actually, there is a lot of chemistry and biochemistry, but for the purposes of this article I’ll keep it minimal) to oxidative stress and how it is managed by the body. Free radicals are oxygen-containing molecules with an uneven number of electrons. Antioxidants are molecules that donate an electron to free radicals; this causes free radicals to be less reactive and more stable. Unstable free radicals can wreak havoc on our bodies.
However, let’s not completely classify free radicals as “bad guys.” Free radicals can help fight off pathogens that could lead to infection. Free radicals are produced constantly by normal cell metabolism within our bodies, such as mitochondria, peroxisomes, endoplasmic reticulum, and phagocytic cells.2,3 For instance, phagocytes (neutrophils, monocytes, macrophages) release free radicals to fight pathogenic microbes that could lead to disease.
The problem is when there is an overabundance of free radicals without enough antioxidants to stabilize them. This is what produces the oxidative stress that can damage DNA and lead to chronic illnesses.
The above explanations are the basics of oxidative stress. How does it affect oral health? What are some of the oral diseases that could be caused by or causing oxidative stress? How can we help patients manage oxidative stress for better oral health and overall health?
Periodontal Disease
Periodontal disease is initiated by an overgrowth of bacteria that harbor in the mouth. When this happens, free radicals are overproduced largely by overactive neutrophils. If these free radicals are not balanced by antioxidants, they begin to cause tissue damage.4
Neutrophils are our first line of defense against bacteria. After the neutrophils are stimulated by a pathogen, they produce oxygen via an oxidative burst that is also referred to as respiratory burst. Oxidative bursts rapidly release free radicals; this is an important part of the immune response as it plays a role in the degradation of the bacteria.4,5,6
Numerous studies have indicated neutrophils in peripheral blood of patients with periodontal disease produced free radicals at a higher rate than those with healthy periodontium. In addition, multiple studies have concluded that periodontal disease could contribute to local and systemic oxidative stress which may explain the comorbidity we see with many systemic diseases and periodontal disease.4
HPV Related Oropharyngeal Cancer
It has been estimated that around two-thirds of oropharyngeal cancers have HPV DNA in them.7 Although there are 100 different types of HPV, several are considered high-risk types. These high-risk types encode two types of oncogenes.
An oncogene is a gene that, under certain circumstances, can transform a cell into a tumor. The two oncogenes found in high-risk HPV are E6 and E7. Factors that exacerbate oxidative stress include other infections, age, epigenetics, chronic infections, and environmental factors. Oxidative stress can lead to DNA damage, which leads to apoptosis. However, cells infected with a high-risk strain of HPV avoid apoptosis with the E6 and E7 oncogenes. These oncogenes are “rescue” cells, leading to mutagenesis and increased cell proliferation. Increased cell proliferation leads to carcinogenic tumors.
In the absence of oxidative stress, the number of oncogenes released by the virus is diminished, which makes it possible for the immune system to clear the virus. Increased free radicals cause an increase in the development of viral carcinogenesis when infected with high-risk HPV strains.8
Dental Caries
A strong correlation has been made between oxidative stress and the initiation of the decay process. More studies are certainly needed to establish a causal link; however, several studies have started the groundwork in establishing a correlation. One study analyzed the total antioxidant status in stimulated and unstimulated saliva in adolescents. It was determined that in this group of individuals an increase in the number of carious lesions was associated with a significant decrease in total antioxidant status in stimulated and unstimulated saliva.9
The proposed mechanism by which oxidative stress could contribute to dental caries has been suggested to be the role of saliva. Saliva has been theorized to be one of the first lines of defense against oxidative stress. The enzymes found in saliva that comprise peroxidase, uric acid, along with several other minor enzymes can counter oxidative stress.10
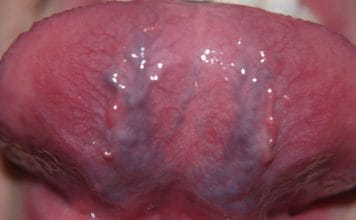
Lichen Planus
The etiology of lichen planus has remained elusive. Recent studies may lead us to a better understanding and possible etiology of this chronic inflammatory disease. Studies measuring the level of malondialdehyde, a marker for oxidative stress, in saliva were significantly higher in patients with oral lichen planus than in healthy control groups.11,12
A systematic review evaluating oxidative stress in oral lichen planus included several studies with results indicating the implication of oxidative stress in the pathogenesis of oral lichen planus. However, in conclusion, the systematic review called for further studies to draw definitive conclusions regarding the correlation between oxidative stress and oral lichen planus.12
Antioxidant Therapy
Antioxidant therapy has been proposed as a possible adjunctive or even primary treatment for oxidative stress. Where do we stand with the science associated with antioxidant therapy? Antioxidant therapy has been studied for its efficacy in the treatment of many diseases related to oxidative stress.
For instance, a study published in March 2018 evaluated the efficacy of antioxidant therapy for the treatment of hypertension. This study concluded that preclinical studies supported the possibility of antioxidant therapy as a useful treatment for hypertension. However, clinical studies provided inconclusive or controversial results.13
As antioxidant therapy applies to periodontal disease, one study used lycopene and green tea extract orally as an adjunctive therapy post SRP. The study concluded, “Lycopene with green tea extract may prove to be a promising adjunctive prophylactic and therapeutic modality in the treatment of gingivitis patients. However, further studies are needed to evaluate the additive effect of antioxidants with routine oral prophylaxis therapy.”
I think it is important to point out a few glaring flaws in this study. We already know SRP is an effective treatment in periodontal disease. The study only followed patients for 45 days post SRP. These flaws leave questions and are the reason we need further studies to get a consensus on the efficacy of antioxidant therapy for periodontal disease.14
This does not mean that antioxidant therapy won’t be an option for dental disease in the future. As a matter of fact, there are many antioxidant drugs approved for medical use currently they include:
- Edaravone use to treat ischemic stroke
- Idebenone used to treat Alzheimer’s disease
- N-Acetylcysteine used to treat Acetaminophen overdose, dry eye syndrome
- A-Lipoic acid used to treat diabetic neuropathy
- Micronized purified flavonoids fraction used to treat persistent venous ulcers
- Silibinin used to treat hepatoprotective, chemopreventive
- Flavocoxid used to treat osteoarthritis
Questions About Antioxidant Therapy
Certain things need to be considered regarding the efficacy of antioxidant therapy and why it may not be effective in certain circumstances. First, oxidative stress may not be the primary cause of a disease. The first hurdle is determining if the oxidative stress initiated the disease, or if it is only after tissue damage that it is detected.
Second, oxidative stress may not be the only cause of the disease. There may be other pathogenic processes that need to be addressed, as well. Not addressing the other pathogenic pathways could make antioxidant therapy seem ineffective.
Third, it is important to consider pharmacogenomics in the testing of antioxidant therapy. Each patient may react differently to medicaments. Knowing the patient’s status where oxidative stress is concerned, i.e., does the patient have elevated markers to indicate oxidative stress? If the patient does not have elevated markers for oxidative stress, then clearly there will be no change with antioxidant therapy.
Fourth, the wrong antioxidant may be used. Studies have shown certain antioxidants are beneficial for different areas of oxidative stress. For example, if the antioxidant administered has low bioavailability, it will not be effective because the antioxidant may be metabolized before it has an opportunity to reach adequate plasma concentrations, rendering it useless.
Many antioxidants have poor target specificity. This is an important aspect as it applies to oral health. Many antioxidants work systemically and do not reach target organs, so antioxidant therapy may reduce oxidative stress overall, yet we may not see results in the damaged tissue such as that of periodontal disease.15
With advancements in science, we may one day see targeted antioxidant therapy. As it stands, we have some great studies indicating oxidative stress as a contributing factor to many oral diseases. Although we don’t currently have an evidence-based treatment yet, stay tuned and up to date on new research as it emerges in the area of antioxidant therapy. I am quite hopeful for the future of dentistry and the science that will lead us to treatment options for patients with oxidative stress-induced oral and systemic diseases.
Now Listen to the Today’s RDH Dental Hygiene Podcast Below:
References
- Kumar, J., Teoh, S.L., Das, S., Mahakknaukrauh, P. Oxidative Stress in Oral Disease: Understanding Its Relation with Other Systemic Diseases. Front Physiol. 2017; 8: 693. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5603668/
- Lobo, V., Patil, A., Phatak, A., Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn Rev. 2010 Jul-Dec; 4(8): 118-126. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3249911/
- Phaniendra, A., Jestadi, D.B., Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Impact in Various Diseases. Indian J Clin Biochem. 2015 Jan; 30(1): 11-26. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4310837/
- Wang, Y., Andrukhov, O., Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front Physiol. 2017; 8: 910. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5693842/
- Yang, P., Huang, S., Xu, A. Chapter 8 – Oxidative Burst System in Amphioxus. Amphioxus Immunity, Academic Press. 2016. Pages 153-165. Retrieved from https://www.sciencedirect.com/science/article/pii/B9780128499030000087
- Slauch, J.M. How Does the Oxidative Burst of Macrophages Kill Bacteria? Still an Open Question. Mol microbiol. 2011 May; 80(3): 580-583. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3109634/
- Cleveland Clinic. Oropharyngeal Human Papilloma Virus (HPV) Infection. Retrieved from https://my.clevelandclinic.org/health/diseases/15010-oropharyngeal-human-papilloma-virus-hpv-infection
- Williams, V.M., Filippova, M., Filippov, V., Payne, K.J., Duerksen-Hughes, P. Human Papillomavirus Type 16 E6* Induces Oxidative Stress and DNA Damage. J Virol. 2014 Jun; 88(12): 6751-6761. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4054338/
- Krawczyk, D., Błaszczak, J., Borowicz, J., Mielnik-Błaszczak, M. Life style and risk of development of dental caries in a population of adolescents. Annals of Agricultural and Environmental Medicine. 2014; 21(3): 576-580. Retrieved from http://www.aaem.pl/Life-style-and-risk-of-development-of-dental-caries-in-a-population-of-adolescents,72158,0,2.html
- Pani, S.C. The Relationship between Salivary Total Antioxidant Capacity and Dental Caries in Children: A Meta-Analysis with Assessment of Moderators. J Int Soc Community Dent. 2018 Sep-Oct; 8(5): 381-385. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/?term=The+relationship+between+salivary+total+antioxidant+capacity+and+dental+caries+in+children%3A+A+meta-analysis+with+assessment+of+moderators
- Rekha, V.R., Sunil, S., Rathy, R. Evaluation of Oxidative Stress Markers in Oral Lichen Planus. J Oral Maxillofac Pathol. 2017 Sep-Dec; 21(3): 387-393. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5763861/
- Mishra, S.S., Uma Maheswari, T.N. Evaluation of Oxidative Stress in Oral Lichen Planus using Malonaldehyde: A systemic Review. Journal of Dermatology & Dermatologic Surgery. 2014 Jan-Jul; 18(1-2): 2-7. Retrieved from https://www.sciencedirect.com/science/article/pii/S2210836X14000037
- Daniela, S., Nicola, D., Bruno, T., Guido, I. The Antioxidant Therapy: New Insight in the Treatment of Hypertension. Front Physiol. 21 March 2018. Retrieved from https://www.frontiersin.org/articles/10.3389/fphys.2018.00258/full
- Tripathi, P., Blaggana, V., Upadhyay, P., Jindal, M., Gupta, S., Nishat, S. Antioxidant Therapy (Lycopene and Green Tea Extract) in Periodontal Disease: A Promising Paradigm. J Indian Soc Periodontol. 2019 Jan-Feb; 23(1): 25-30. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6334550/
- Firuzi, O., Miri, R., Tavakkoli, M., Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr Med Chem. 2011; 18(25): 3871-88. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21824100